Bond Angle of Cyclohexane
Structural formula shows the minimal detail that shows the arrangement of atoms in a. Given a set of atoms and a vector r describing the atoms positions one can introduce the concept of the energy as a.

Cyclohexane Chair Flip Summary Of How To Draw A Ring Flip Mcat Study Chemistry Student Learning
Based on previous synthetic methods in our lab as shown in Scheme 2 6519 g propionic acid 25 g cyclohexane 01 ml 2-methylpyridine and 08 g heteropolyacid IL catalyst were mixed in a 500 ml three-necked flaskThen 25 g H 2 O 2 50 was added slowly through a 100 ml constant pressure funnel at 65 C 54 kpa with a magnetic stirring for 4 h.
. Angle of incidence 090. Typically bond flange widths should range in size from 16 to 25 mmA minimum flange width of 6 mm may be used on smaller parts such as spoilersA minimum of 3 mm clearance from the tangent should be allowed between the edge of the inner panel and the. Angle ring strain present in the compound when the bond angle between two bonds differs from the question_answer Q.
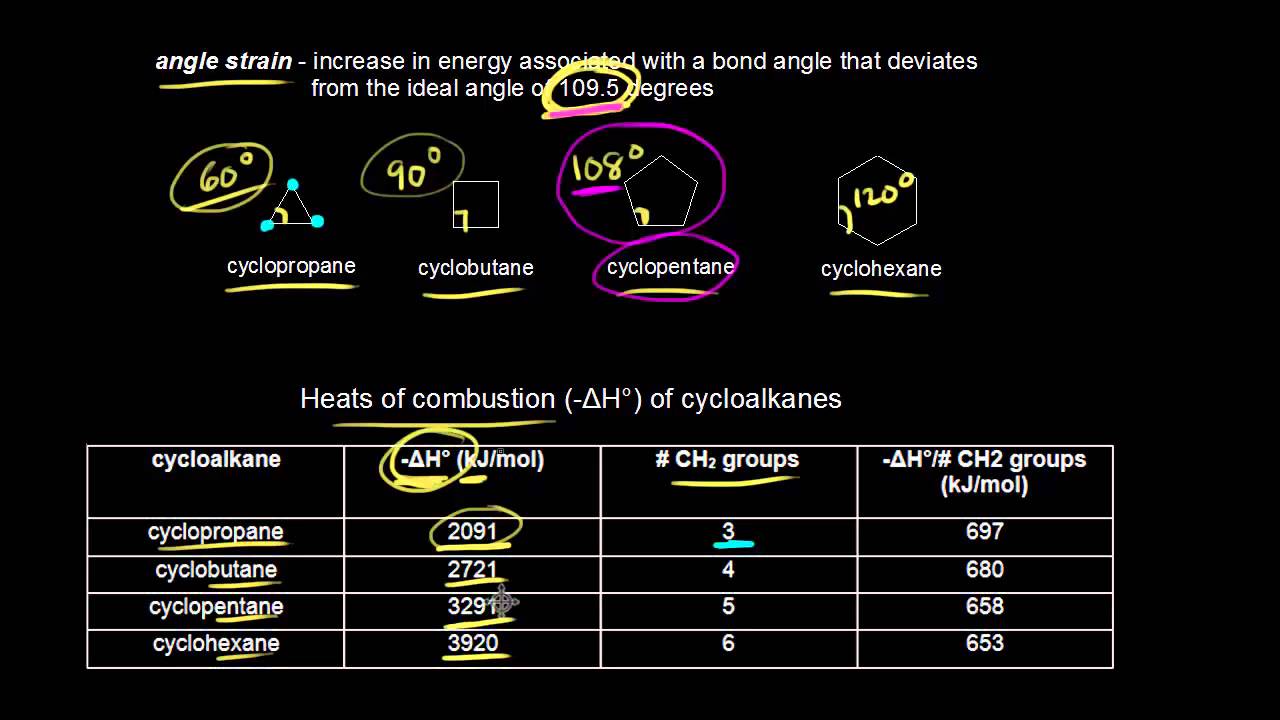
Small rings such as three and four membered rings have significant angle strain resulting from the distortion of the sp 3 carbon bond angles from the ideal 1095º to 60º and 90º respectively. Molecular geometry and mathematical interpretation The geometry of a set of atoms or molecules can be described by Cartesian coordinates of the atoms or internal coordinates formed from a set of bond lengths bond angles and dihedral angles. The transmission electron microscopy TEM and high-angle annular dark-field scanning TEM HAADF-STEM images Fig.
1c of the p-Pt 45 Ni 55 showed an octahedral shape with an average edge length. A few other uses of saturated hydrocarbons are listed below. 発音を聞く 例文帳に追加 cat scancatスキャンcomputerized axial tomography scanコンピュータx線体軸断層撮影法computerized tomographyコンピュータ断層撮影ct scanctスキャンとも呼ばれる.
Cyclohexane C 6 H 12 Cyclopropane C 3 H 6. This angle strain often enhances the chemical reactivity of such compounds leading to ring cleavage products. What are the Uses of Saturated Hydrocarbons.
Also called cat scan computerized axial tomography scan computerized tomography and ct scan. The molecular geometry is given in parentheses. Indicate whether each of the following molecules is polar or nonpolar.
In small-ring cyclic compounds ring strain can be a major contributor to thermodynamic instability and chemical reactivity. Among these angle strain and eclipsing strain bond orientation are severe in small rings such as cyclopropane and. Adolf von Baeyer received a Nobel Prize in 1905 for the discovery of the Baeyer strain theory which was an explanation of the relative stabilities of cyclic molecules in 1885.
P-polarized R P S-polarized. Angle strain Baeyer strain Alkanes. Bond flanges should be designed to be as narrow as possible without sacrificing the structural integrity of the assembly.
The most common cyclic compounds have five or six carbons in their ring. Since cyclopropane has a carbon-carbon bond angle of 60 o it has the highest ring strain among all cycloalkanes. 57 710-717 1940 Data CSV - comma separated TXT - tab separated Full database record.
Cyclohexane is clearly the most stable lower potential energy of the four isomers depicted. A hydrogen bond or H-bond is a primarily electrostatic force of attraction between a hydrogen H atom which is covalently bound to a more electronegative donor atom or group Dn and another electronegative atom bearing a lone pair of electronsthe hydrogen bond acceptor Ac. Such an interacting system is generally denoted DnHAc where the solid line denotes a polar.
Alkanes are widely used as fuels heating oils and solvents. In alkanes optimum overlap of atomic orbitals is achieved at 1095. What reactant would transform the molecule from the top left to the top right.
Index of refraction of methane in the infra-red and the dipole moment of the CH bond Phys. PH2Cl trigonal pyramidal with P at the apex bSO3 trigonal planar with S in the center position cCH2Cl2 tetrahedral with C in the center position dCCl4 tetrahedral with C in the center position.

Stability Of Cycloalkanes Organic Chemistry Books Organic Chemistry Chemistry

Cyclohexane Conformations Master Organic Chemistry Organic Chemistry Teaching Chemistry Chemistry Lessons

Cyclohexane Conformations Master Organic Chemistry Organic Chemistry Chemistry Chemistry Labs

Convert Newman Projection Of Cyclohexane To Bond Line Chemistry Textbook Chemistry Lessons Study Chemistry
0 Response to "Bond Angle of Cyclohexane"
Post a Comment